History of VLPs: (As compounds of advanced vaccine Strategies)
Bioprocessing of VLPs | Gofrixty | Previous to 1969 anti-viral vaccines were either derived from inactivated viruses such as rabies vaccine, attenuated strains of pathogenic wild type viruses such as (yellow fever or poliovirus) or they were using non-pathogenic viral strains to persuade a defending immune response against pathogen family members like a vaccine of smallpox containing harmless vaccinia virus. Since then particles based on HBsAg were firstly revealed in the blood samples of patients infected with HBV and soon were developed into an intoxicating vaccine against similar viral infections.
Subviral particles have hurriedly found their way into present concepts of vaccine design. This unique nature of virus-like particles presents an inbuilt benefit over soluble antigens. They have the potential of stimulating innate and adaptive immune responses. These soluble antigens have been shown to be unsuccessful in many vaccine approaches due to feeble immunogenicity. VLPs are not harmful and do not replicate, they also embody a safer option to attenuated viruses, which have succeeded to guard against yellow polio, influenza and yellow fever virus etc.
Introduction Bioprocessing of VLPs
VLPs are greatly ordered spheres that self-assemble from virus-derived structural antigens. These are the most stable sub-viral particles that acquire exceptional adjuvant characteristics able of inducing inherent and associated immune responses. Commercially available virus-like particle-based vaccines have been very successful in protecting humans from HBV and also HPV disease. Presently VLPs are explored for their potential to combat cancer and another infectious disease.
Design of VLP has been obtained from HIV, such VLPs show potential for current AIDS approaches. Because of their exceptional characteristics, virosomes and VLPs have recently gained importance in the field of nanobiotechnology for the production of novel biomaterials. Viruses contain a protein coat that is made of many subunits and at times they are bounded by a lipid layer resulting from the cellular membranes of the host. They have developed many shapes and replication tactics in order to stay alive in the host environment. Though not all viruses contain nucleic acid which is mandatory to begin infection, virus-like particles are able to enter the target cells and they persuade host immune response from viral infection.
This experience has been profoundly described for those cells which were infected with HBV, that mass creates empty 22 nm particles exclusively composed of small hepatitis B virus surface antigen (HBsAg). Fast-growing information of virus-like particle functions and organization combined with the actions in genetic engineering have opened multiple opportunities to develop virus-like particles for a variety of fields of molecular biology. Particularly their natural immunogenic properties make them more attractive candidates for vaccine strategies.
Immunogenic properties of VLPs:
In contrast with soluble antigens, viruses like particles are able of inducing strong cellular and humoral responses as direct immunogens. Virus-like particle size appears to be favourable for uptake by DC through endocytosis and macropinocytosis that play a vital role in activating inborn and adaptive immunity. Data shows that Gag virus-like particles per se have many immunogenic epitopes that are able of stimulating cellular immunity through both, the MHC class-I and MHC class II pathways.
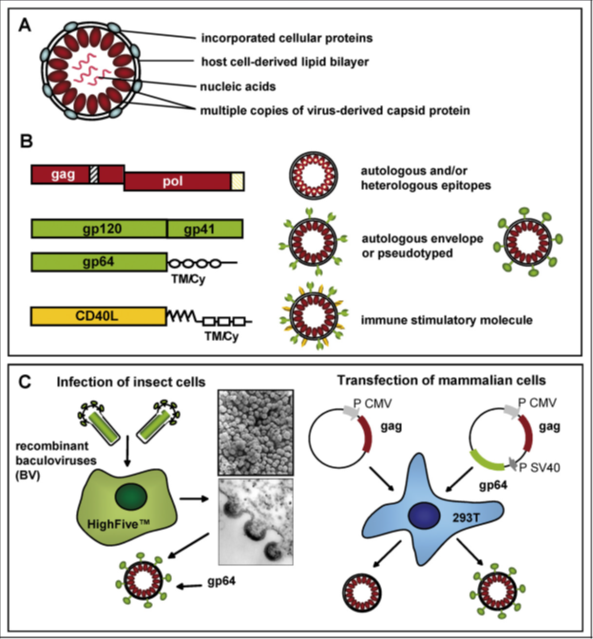
Due to the cyclical particle arrangement, uptake of a single virus-like particle supplies thousands of contained epitopes into the processing and presentation machinery of APCs. This method is thought to be supported by the lipid nature of virus-like particles. All these statements with new findings that pseudotyping of virus-like particles with envelope proteins like VSV-G, thus improving uptake by receptor-dependent fusion, increase epitope presentation through the exogenous MHC class-I pathway and successive CTL induction.
In my opinion, all well-known VLPs are confirmed to be strong stimulators of inborn immunity. An extensive prospective of VLPs to activate dendritic cells by activating the expansion of many populations of immune cells in vivo has just been assiduously signalled. The development of such a vaccine has many safety concerns in view of the associated regulatory complications. Fighting the well-known viruses such as HPV, HBV, etc are continually being carried over to recently rising viral diseases, for which virus-like particle-based tactics serve as first tryout tools to make a defensive vaccine.
Mechanisms and mode of action of VLPs
Following are the steps involved in reputed mechanisms of virus-like particle-mediated incentive of inherent and associated immune responses:
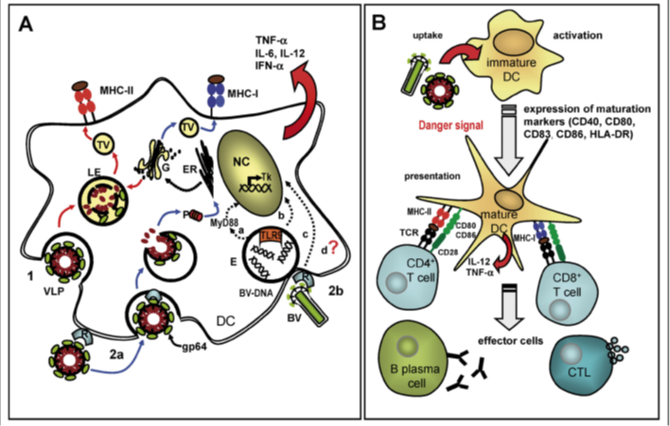
A) Model for the establishment of DCs by baculo-derived virus-like particle preparations. Virus-like particles are taken up by DCs through endocytosis (1) directing Ag processing in LE and appearance by the major histocompatibility complex class-II pathway, or through receptor-mediated fusion carried out by gp64 (2b) results in proteasomal Ag processing in the cytoplasm and following presentation on MHC class-II. Baculoviruses are taken up via gp64-mediated fusion (2b).
Hazardous signals from CpG-rich BV-DNA are accepted by endosomal TLR9 and transmitted by means of a MyD88-dependent or self-regulating signalling pathway which results in activation of transcription and production of inflammatory cytokines and type 1 interferon. A toll-like receptor 9-independent path of DNA detection inducing the production of IFN. Furthermore, baculovirus-derived apparatus other than DNA might leads to activation of the dendritic cells. Golgi apparatus, endoplasmic reticulum, nucleus, TV act as transport vesicles.
B) It shows the VLP-mediated maturation of dendritic cells. Uptake of virus-like particle/baculovirus activates DC through danger signals and these results in up-regulation of dendritic cell maturation markers. Mature dendritic cells present virus-like particle-derived Ag to immature CD4+ and CD8+ T cells through MHC class-I and class-II. The discharge of cytokines by dendritic cells stimulates segregation into B and T effector cells result in the release of antibody cytotoxic T cell response.