Introduction Recombinant Protein
Recombinant protein is a modified form of protein, which is generated in many ways to produce large quantities of proteins, alter gene sequences and produce useful commercial products. The formation of recombinant protein is carried out in specialized vehicles known as vectors. Recombinant protein is a protein encoded by a gene — recombinant DNA — that has been cloned in a system that supports the expression of the gene and the translation of messenger RNA. Proteins co-expressed in bacteria will not have post-translational modifications, e.g. phosphorylation or glycosylation. Eukaryotic expression systems are required for this. Efficient strategies for the production of recombinant proteins are gaining increasing importance, as more applications that require high amounts of high-quality proteins reach the market.
Recombinant proteins have been utilized as tools for cellular and molecular biology. Today, more than 75 recombinant proteins are used as pharmaceuticals, and more than 360 new medicines based on recombinant proteins are under development. The impact of the production of recombinant proteins has also extended to the development of bioinsecticides, diagnostic kits, enzymes with multiple applications, and bioremediation processes, among many others. In particular, areas such as detergent production and food processing have been among the most notable success. Even when hundreds of proteins are produced at a commercial scale, the production of recombinant proteins still imposes a challenge in many cases. In this assignment, typical problems encounter during recombinant protein production are reviewed and strategies to solve them and increase productivity.
1. Loss of Expression
A necessary condition for sufficient recombinant protein production is the efficient expression of the gene of interest. However, expression can be lost due to structural changes in the recombinant gene or loss of the gene from host cells. Plasmid-Based Systems Plasmids are extrachromosomal self-replicating cytoplasmic DNA elements that are found in prokaryotes and eukaryotes. They have been used as molecular tools for recombinant genes since the dawn of genetic engineering.
Plasmid-based expression
It is the most popular choice when we use prokaryotes as hosts, as genetic manipulation of plasmids is quite easy. Furthermore, gene dose that normally depends on plasmid copy number is higher than when the recombinant gene is inserted into the host’s chromosome. Plasmid copy number is an inherent character of each expression system and normally depends on the plasmid, the host, and the culture conditions. Plasmid copy numbers can range from a few up to 200. The metabolic load increases when there is an increase in the size of the insert, temperature, level of expression, recombinant protein yield, and toxicity of the protein expressed toward the host. Such kind of metabolic load sometimes results in a decrease in the growth rate of plasmid-containing cells. Consequently, the growth rate decreases and faster-growing plasmid-free cells ultimately overtake the culture. Loss of plasmid is the main cause of reduced recombinant protein productivity in plasmid-based systems. This is called plasmid segregation instability. Another factor that adds to plasmid instability is plasmid multimerization. As plasmid copies have the same sequence, they can recombine forming a single dimeric circle that has two origins of replication. This results in lesser independent units being separated between daughter cells, and subsequently, plasmid loss can increase. In addition, cells that bear multimers grow slower than those bearing monomers, even at the same copy numbers. Other factors that influence plasmid stability are plasmid size (larger plasmids are less stable) the presence of foreign DNA, rate of cell growth, nutrient availability, temperature, and mode of culture. Several natural mechanisms exist to guarantee plasmid survival in cell populations. For example, low-copy-number plasmids assurance their persistence by multimer resolution through site-specific recombination systems or via active partition mechanisms, such as the par sequences. Genes responsible for both mechanisms have been incorporated in such man-made plasmids that increase their stability
Chromosomal Integration
Chromosomal integration of the gene of interest is a better alternative to overcome problems of expression stability in plasmid-based systems. Chromosome integration is particularly suitable for the metabolic engineering of the host. However, many disadvantages over plasmid-based systems exist for recombinant protein production. Appropriate integration of a foreign gene in the chromosome is labour-intensive and very time-consuming. But the recombinant cells obtained are able to grow in the absence of antibiotics with no reduction of recombinant protein yields. This approach also had the advantage of not infringing patents. Still, a major problem faced with chromosomal integration is the chance that the gene of interest will become inserted into an inactive region of chromatin. Among the many strategies used to overcome such a problem is the use of locus control regions (LCRs), which ensures transcriptional regulation of the transgene.
Viral Vectors
Another easy and very effective way of delivering the gene of interest is via viral vectors. Viruses have evolved to transfer their genetic material to the host in an efficient and non-destructive way. Some viral vectors, such as retroviruses, facilitates the integration of the viral genome into the cell’s chromosome. Many others are used for temporary expression. In these cases, recombinant protein production occurs only during particular stages of the life cycle of the virus. The simplicity of this expression method makes it useful for production in higher eukaryotes, as obtaining stable recombinant animal cells may be a tiring and long process. For example, the insect cell baculovirus expression vector system (BEVS) is used to commercially produce many recombinant proteins.
Keep Reading:
- Bioprocessing of VLPs used in vaccine production
- What is health system?
- What is pooled risk in terms of healthcare insurance system?
- 4 helpful tips to avoid toxic people in your life in 2021
- Tuberculosis in Pakistan and its impact on an individual’s life
- Biofloc fish farming in Pakistan
Watch Our Latest Videos:
- Naila Shamal | Bride from Mardan demanded Rs 100k worth of books in Haq Mehr
- Top 10 places to visit in Islamabad this year
Moreover, BEVS is especially feasible for the production of vaccines. Recombinant gene expression from viral vectors comprises special issues that are different from those of plasmid- or of chromosome-based systems. The use of viral vectors is involving a process with two different phases: first, cells are grown to the desired cell density, and then they are infected with the virus of interest. In addition, a virus-free product must be ensured for most applications; thus, particular considerations are needed during purification procedures. One of the most important confines of expression systems based on viral vectors is the quality of the viral stock. Serial in vitro passaging of stocks can result in the emergence of mutant viruses that are known as defective interfering particles (DIP). The genome of DIP has several deletions that make their replication faster than that of intact viruses. Therefore, DIP fights for the cellular machinery and can severely decrease recombinant protein yields. As DIP replication needs a helper virus, in this case, the complete virus, their amassing can be avoided by using multiplicities of infection (MOI) less than 0.1 plaque-forming unit (pfu) per cell.
Posttranslational Processing
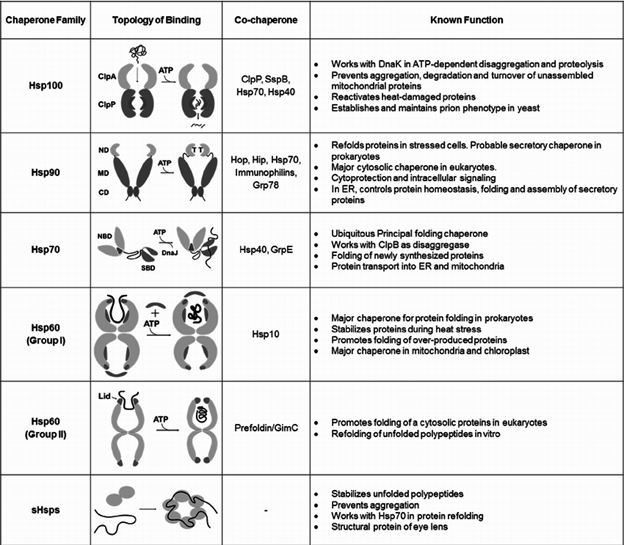
Folding, Aggregation, and Solubility Protein folding is an intricate process in which two kinds of molecules play an important role: foldases, which speed up protein folding; and chaperones, which protect from the formation of non-native insoluble folding entities. On occasions, folding does not proceed properly. This results in misfolded proteins that gather in intracellular aggregates known as inclusion bodies. One of the main reasons for incorrect protein folding is cell stress that may be caused by heat shock, nutrient deficiency, or some other stimuli. Cells answer to stress by enhancing the expression of various chaperones, some of them of the hsp70 and hsp100 families. Production of inactive proteins shows an energetic exhaust and metabolic load while amassing of inclusion bodies can also cause structural strains to the cell. For instance, many human pathologies, such as Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease, are characterized by intracellular protein aggregation and accumulation. During the production of heterologous protein, high rates of expression are needed. Accordingly, the formation of an inclusion body is more likely to occur during the production of recombinant protein. Aggregation saves proteins from proteolysis and can smooth the progress of protein recovery by simply breaking the cells and centrifuging the inclusion bodies. In addition, when the expressed protein is harmful to the host, its deleterious effect can be disallowed by producing the heterologous product as inclusion bodies. Accordingly, inclusion body formation not only is wanted, but also can be promoted through molecular biology and/or operation strategies, such as the use of protease-lacking strains, culturing at high temperatures, or designing suitable fusion peptides and amino acid sequences through protein engineering approaches. Protein engineering can also reduce aggregation changing the level of hydrophobic regions or using fusion proteins are two successful strategies
Proteolytic Processing
Signal peptides, required to direct proteins to the various cellular compartments, must be cleaved to get a functional protein. Upon membrane translocation, the signal peptide is detached by a signal peptidase complex that is membrane-bound to the endoplasmic reticulum in eukaryotes or to the cellular membrane in prokaryotes. Inappropriate removal of the signal, the peptide may result in protein accumulation and retention within incorrect compartments, such as the endoplasmic reticulum. Ultimately, the yields of secreted proteins can be significantly reduced. To solve this problem, the E. coli signal peptidase I and the Bacillus subtilis signal peptidase are being overexpressed in E. coli and insect cells.
Glycosylation
Glycosylation is a very complex posttranslational modification that needs several consecutive steps and involves many enzymes and substrates (Fig. 2)
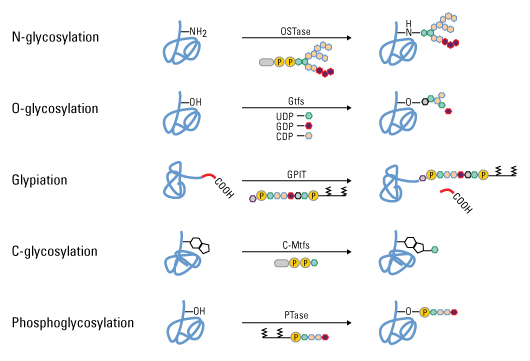
It usually occurs in the endoplasmic reticulum and Golgi apparatus of eukaryotic cells, although N-glycosylation has been noticed in proteins produced by bacteria. Three types of glycosylation is there: N-(glycans linked to an Asn of an AsnXaaSer/Thr consensus sequence, where Xaa is any amino acid), O-(glycans linked to a Ser or Thr), and C (attached to a tryptophan) like sequence. N-linked glycosylation is the most studied and is thought as the most relative for recombinant protein production. In many cases, glycosylation tells about protein, folding, solubility, antigenicity, localization, biological activity, stability and circulation half-life. Improper Glycosylation may activate immune responses when present in proteins for human or animal use. Therefore, proper glycosylation is especially required for recombinant proteins to be utilized as drugs. First, the synthesis of the dolichol phosphate oligosaccharide can affect the level of glycosylation. On the other hand, a decreased pool of sugar nucleotides, the activated sugar donors are required for oligosaccharide synthesis, limits the formation of the G3M9N2Dol PP precursor (where G is glucose, M is mannose, and N is N-acetylglucosamine) and decreases the glycosylation site possession. To improve such a problem, sugar nucleotide precursors have been added to the culture medium.
2. Other Posttranslational Modifications
Other posttranslational modifications, such as phosphorylation, myristoylation, sulfation, palmitoylation, isoprenylation, C-terminal amidation, β-hydroxylation, and methylation, are less common than glycosylation but are important for certain recombinant proteins.
Transport and Localization
Recombinant proteins may be transferred to different cellular compartments by signal peptides or through fusion proteins. Intracellular amassing often results in high protein amounts and allows easy recovery of concentrated protein along with cells. Nevertheless, purification of the product from the protein-rich cell extract may be tedious. Accordingly, concentration procedures, such as ultrafiltration, are always used before other purification stages when working with secreted proteins. Aggregation in the periplasm often results in soluble and correctly folded proteins, whereas cytoplasmic localization results in an inactive and insoluble product. Small proteins are susceptible to proteolysis and should be produced in E. coli as inclusion bodies. Apart from intra- or extracellular accumulation, some applications may need recombinant proteins to be targeted to the cell membrane, usually via fusion proteins.
3. Bioengineering Approaches to Solve Common Problems Associated With Heterologous Gene Expression
Bioprocess engineering plays an important role when the aim of recombinant protein production is to get as great amounts as possible of a high-quality product. Bioprocess conditions affect not only the quantity of protein produced but also its solubility and its posttranslational modifications. The biology of the host, and the molecular biology tools used for its modification, should be considered when defining bioprocess conditions.
Induction Strategies
Recombinant genes can be placed under many promoters. The promoter selected will decide whether gene expression is constitutive or inducible. Constitutive gene expression may enhance plasmid instability because the metabolic load of recombinant protein production is continuously present. Thus, constitutive promoters are normally selected when recombinant gene expression does not significantly affect the growth rate of the host. In many cases, the best conditions for cell growth are very different from those for recombinant protein production. In these cases, inducible systems are preferable i.e., systems in which induction is performed after a particular cell density has been obtained. Induction may depend on the lack of a nutrient and/or the addition of an alternative nutrient that turns on specific molecular machinery, such as the lac operon. Other inducers include osmolarity, or temperature shifts, pH, anaerobiosis, antibiotic addition. Induction should be simple, economical, and efficient. In addition, the inducer should not have harmful effects on cell viability and quality of recombinant product, and should not cause hindrance in downstream operations. Finally, the chosen system should be effectively repressed in the absence of the inducer. Industrial recombinant protein production needs additional considerations of the type of inducer used. Among these are the deficient mass, heat, and momentum transfers often seen in large-scale bioreactors. Thus, up to 16 min would be needed for the inducer to be homogeneously distributed in the reactor under this extreme condition. This can be solved by using several feeding ports if the inducer is a chemical poured into the vessel. However, such an approach cannot be used for another type of induction, such as temperature changes. Decreasing or increasing the temperature of a large scale vessel can be very expensive and ineffective sometimes. The rate of temperature change can also affect recombinant protein yield.
Growth Control
Growth rate affects many parameters that decide recombinant protein accumulation rate. Among them is the fraction of substrate utilized for cellular protection, RNA polymerase activity, ribosome number, plasmid copy number, plasmid stability, plasmid multimerization, and the distribution of cells in the cell-cycle phases. It is possible to control recombinant protein production through growth rate. The growth rate can be changed through nutrient availability. The main carbon or nitrogen source can be maintained at a preset concentration to obtain the desired growth rate. Such exploitation can be achieved through fed-batch or continuous cultures. Dissolved oxygen, an important nutrient for aerobic cells, can also be used to control growth rate. Temperature also affects growth rate by changing the rate of the reactions that occur in the culture vessel. It should be noted that all these factors can have additional special effects besides changing the growth rate. For instance, decreasing the growth rate by limiting nutrient concentration may decrease the production of undesirable metabolites by increasing metabolic efficiency. Molecular biology approaches can also be utilized to manipulate growth rates.
4. Bioreactor and Operation Strategies
The main purpose of a bioreactor, besides containment, is the control of environmental parameters in predefined values. The number of parameters that can be manipulated depends on the structure of the bioreactor. It can range from one parameter, when static culture flasks are introduced in an incubator, to many parameters in a fully instrumented vessel. Among the conditions that can be controlled are dissolved oxygen, temperature, pH, agitation rate, redox potential, dissolved carbon dioxide, cell growth, cell concentration, cell, substrate concentration, volume, inlet gas flow and composition, pressure, fluid dynamics, and power input. The environment results not only controlling the action of process parameter controlled by the operator but also from the direct communication of cells with their environment. The environment interacts with many steps of the recombinant protein production process, namely transcription cell growth, translation, cellular metabolic state, and posttranslational modifications. From the importance of the environment, it can be seen that bioreactors have a massive potential for enhancing recombinant protein productivity. Of the factors listed above, dissolved oxygen tension (DOT) has got special attention, because oxygen has low solubility in water and is difficult to transfer to the culture broth. The problem starts at very high cell concentrations, as higher amounts of oxygen must be sending to the culture medium to fulfil demand. Cultures need to be fully aerated and homogeneous to avoid alcoholic or acid fermentation in bacteria, yeast, and animal cell cultures. Accordingly, bioreactors are designed to enhance the oxygen transfer rate (OTR) as much as possible. The bioreactor operation mode is another way to control the environment. Fed-batch cultures are used for increasing cell concentration and getting high product titers). The control of nutrient concentration can enhance metabolic efficiency. For example, maintenance of low glucose concentration can be used to evade the Crabtree effect. On the other hand, nutrient-limited cultures are more severely affected by the metabolic burden of foreign gene expression. Glucose, phosphate, magnesium or oxygen limitation decrease plasmid stability. At the same time, the carbon-to-nitrogen ratio also affects plasmid loss and the burden that plasmids impose on cells. Bioreactor operation mode also affects plasmid stability.
5. Specific Problems and Their Solutions in Different Expression Systems Recombinant protein:
Production needs incorporated bioprocesses that include considerations ranging from molecular biology to downstream processing. Under this view, the host undoubtedly has a considerable role. Many characteristics of the product are endowed by the host and are affected by protein concentration and site of aggregation. As a rule of thumb, the most simple host expression system that gives the required qualifications should be chosen for recombinant protein production. Animal cells, fungi, yeast, and bacteria are normally used nowadays for the expression of recombinant products.
Prokaryotes
The Gram-negative bacterium E. coli was the first organism used for the production of recombinant human proteins. It is still extensively used for industrial applications. E. coli is easy to grow to high cell densities (over 100 g/L) and has simple nutritional needs that can be fulfilled with fully defined simple media. Regardless of its proven success, recombinant protein production in E. coli has several disadvantages that have been addressed through different approaches. E. coli is usually not capable of effectively producing very long or short proteins, although the successful expression of a 210 kDa protein has been achieved. Proteolytic cleavage and disulfide bond formation rarely occur, and posttranslational medications, including glycosylation, acylation, and isoprenylation, are not performed. In addition, bacteria have pyrogens and endotoxins that must be eliminated from proteins to be injected into animals or humans. Other concerns about the expression of recombinant proteins in E. coli include protein solubility and protein purification. Most of these problems have been approached through genetic modifications. The problem of expression levels traditionally has been solved by using strong promoters and/or superseding on the pathways that include possible rate-limiting steps—namely, a novel “metabolic optimization” methods can be used to newly regulating the expression of genes in particular pathways that change the final product yield, as well as the expression of the gene of interest. Fine-tuning can be obtained by utilizing artificial promoters with different strengths.
Yeast and Fungi
Yeasts have been used by humans since the Neolithic age. Their multiple applications in the food industry and for single-cell protein production has taken yeast fermentations to the largest volumes ever used. The yeast Saccharomyces cerevisiae was the first yeast species to be manipulated for recombinant protein expression and many proteins have been produced in it. Like other eukaryotes, yeasts are also capable of performing most posttranslational processing like mammalian cells. However, extracellular proteases and some differences in glycosylation in proteins expressed in yeast, compared to those of mammalian cells, limit their use. N-glycosylation of proteins produced by yeasts are high-mannose (with more than 3 mannose residues) or hyper mannose (more than 6 mannose residues) types, with terminal α-1,3 linkages. These forms are very immunogenic to mammals. Unchanged yeasts are suitable for the production of proteins that do not need mammalian-type glycosylation and are resistant to proteases. Industrial application of methylotrophic yeasts started when they were used for single-cell protein production. Very large fermentations of methylotrophic yeasts were performed in the 1970s. Recombinant protein concentration has been enhanced by combining the gene of interest with genes of fungal origin. Fungi produce proteases; this limits their use for recombinant protein production. Promoting growth in pellets and controlling pH can decrease protease activity more than fourfold. Such methods have been used for the commercial production of chamois.
Animal Cells
Animal cells have been cultured in vitro for more than a hundred years. For a long time, they have been used for the production of viruses as vaccines, or for the production of endogenous proteins, such as interferon. The first recombinant proteins approved for human use were produced in bacteria, but of 33 products approved by the FDA, 21 are produced by animal cells. It is thought that this situation will continue as more proteins with pharmaceutical applications have complex glycosylation that cannot be produced in prokaryotes or lower eukaryotes. However, successful recombinant protein production in animal cells had to overcome many hurdles, such as the cellular weakness and the complex nutritional requirements of cells.
Mammalian cells
Recent advancement in gene transfer is the use of baculovirus vectors in cultured mammalian cells. Baculoviruses used with this purpose have promoters that are effectively transcribed in mammalian cells, such as those from the Rous sarcoma virus or cytomegalovirus. Other issues that arise when expressing proteins in mammalian cells can be resolved through cell engineering. For example, when large scale production is required the cells suffer metabolic pressures, such as oxygen depletion and toxic metabolite accumulation, which ultimately affect final yields.
Explained above are some of the problems encountered during recombinant protein production and their possible solutions.